By Andrew L. Smith, MD
Heart failure is a pathophysiologic state in which the heart is unable to deliver adequate blood to the body, either at rest or with activity, and also fails to maintain normal intracardiac pressures. Heart failure is a syndrome of signs and symptoms that often progresses without appropriate intervention. To patients, “heart failure” is a distasteful term because it implies sudden doom. However, it is more commonly a chronic condition that limits activity, causes anxiety from an inability to breathe and may result in abdominal bloating, leg swelling, sleep difficulty, dizziness, depression and fear as well as financial difficulty.
For a variety of reasons, heart failure forces people to live life differently from before their diagnosis. On a national scale, heart failure is a public health issue with major economic implications.
To the clinical care team, a successful program requires a chronic disease management model that involves proper clinical assessment, titration of guideline-directed medical therapies, patient self-management strategies and the ability to make interventions when needed, as defined by the patient’s status. This implies a continuous care model that is not constrained to episodic office visits.
Heart failure touches our very human condition, including vulnerability, denial, uncertainty, resilience, perceptions and values. How do we put this all together to improve the health of our patients?
We do this by listening to our patients and appreciating what they experience. We also need to understand the pathophysiology of the disease state, use clinical assessment to facilitate medication adjustments, titrate disease-modifying therapies gradually, convince patients of the benefit of taking medications and create a care approach that allows intervention in a timely fashion.
Medication Benefit
Patients treated for heart failure often take more than eight medications at various intervals during the day, resulting in more than 12 pills per day. Many patients do not know the proven benefits of these medications. We prescribe pills to relieve symptoms, improve prognosis and reduce the impact of comorbidities such as hypertension, diabetes mellitus, atherosclerotic disease, atrial fibrillation and dyslipidemia. Information communicated to patients is often on what these medications due pharmacologically, i.e. reduce the workload on the heart, improve blood pressure. Our communications generally fail to relate what they accomplish: prolong life, reduce hospitalizations and improve quality of life.
ACE inhibitors reduce mortality by about 16 percent, beta blockers (carvedilol, metoprolol, bisoprolol) provide an additional 30 percent to 35 percent reduction in mortality beyond the impact of ACE inhibitors. Hydralazine/nitrate has benefit in African Americans. Spironolactone has also shown improved outcomes in a subset of patients with advanced symptoms.
Conversations about medication benefit are increasingly important, particularly when guideline-directed therapy may no longer be accomplished inexpensively. Now we must consider that in patients with HFrEF, sacubitril/valsartan (Entresto®) reduces mortality an additional 16 percent compared to enalapril in patients with ongoing, NYHA Class II-III heart failure symptoms despite standard therapy. Stated another way: this medication doubles the survival impact of ACE inhibitors and does so in patients already on beta blocker therapy. But cost constraints have impacted utilization of this medication, including failure of insurers to pay and/or high co-pays. Discussing financial ability to pay, informing patients of benefits and assisting them through preauthorization has now become a common part of our patient management.
Diet, Lifestyle and Daily Management
Not only is medication adherence important, but changes from the usual American diet are required to avoid excessive sodium loading. Sodium overload in patients with altered regulatory systems results in fluid retention, congestion and clinical instability. Without a good recipe plan, patients often experience trade-offs in taste in order to be less symptomatic. It is important for patients to read food labels and to attempt where possible to find foods that have less than 100mg per serving. Unfortunately, economic constraints and family dynamics may be barriers to achieving this.
A successful self-management plan involves monitoring blood pressure, weight and symptom recognition. Patients play a key role in maintaining their own health. It is helpful to explain to patients that our bodies are not programmed correctly to have an abnormal heart. Sympathetic activation and neurohormonal signals result in release of substances that make us thirsty, cause fluid retention and reduce blood flow to our skin, causing heat or cold intolerance.
The body’s response to heart failure is very similar to the response to dehydration. These responses cause the patient with heart failure to experience thirst and to misinterpret the cause of symptoms as medication side effects. Without proper education and disease-altering medications, patients will naturally do the wrong thing. It is helpful to tell patients with HFrEF that the proven medications help correct the body’s abnormal response.
Symptoms are a Late Indicator of Congestion
Although worsening dyspnea from congestion may be perceived as sudden, numerous pathophysiologic changes occur in the weeks prior to symptom deterioration that may lead to emergency room visits and ultimately hospitalizations. Implantable cardiac devices such as ICDs and cardiac resynchronization pacemakers collect data that gives insight into these changes.
Physiologic sensor data demonstrates increased sympathetic tone as measured by an increase in average daily heart rate, nocturnal heart rate, respiratory rate and by a decrease in heart rate variability. Decrease in pulmonary impedance may indicate lung congestion.
Unfortunately, remote management strategies using single physiologic sensor data have yet to show improved outcomes. 1 A trial using a multisensor approach, Manage HF, is currently underway. 2
Remote Weight Monitoring vs. Direct PA Pressure
Sudden changes in weight may be indicative that the patient with heart failure is retaining fluid. It is common for weight gain to occur prior to heart failure hospitalizations. Daily weight monitoring with diuretic adjustment for changes of 3-5 pounds are generally recommended.
It seems logical that remote patient monitoring through an automated phone system or with electronic scale data transmitting weights to the clinic would improve outcomes. However, to date large randomized trials of remote monitoring of weight or physiologic sensor data has not been superior to traditional management. (Figure 1)
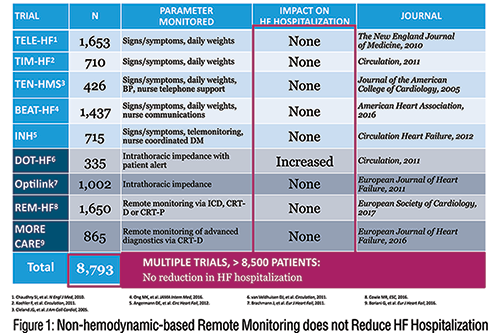
We now have technology that can remotely monitor pulmonary artery pressure and effectively guide early intervention, particularly through diuretic adjustment. The sealed CardioMEMS™ pressure sensor is implanted into a distal pulmonary artery, and instantaneous pressure readings can be accessed and transmitted by the patient from their home. In patients with prior hospitalization and ongoing NYHA class III symptoms, this management system reduced hospitalizations by 33 percent. Patients with preserved and reduced ejection fractions had benefit. 3
Successful implementation of this technology requires a patient who is adherent with transmitting, has diuretic responsiveness and follows instructions on adjusting therapy. It also requires a care team that responds to the transmitted information rather than relying solely on patient symptoms and weight. Cost constraints, insurance coverage and patient selection concerns have impacted utilization of this device in real-world experience. A real-world registry has confirmed the utility of this system.
Figure 2 shows the CardioMEMS™ system.
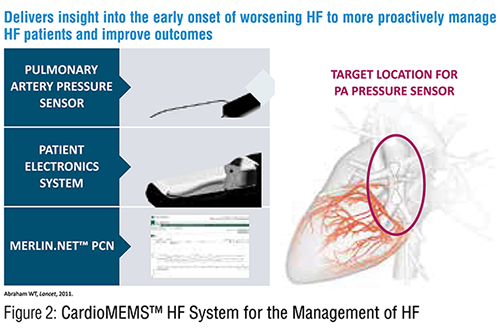
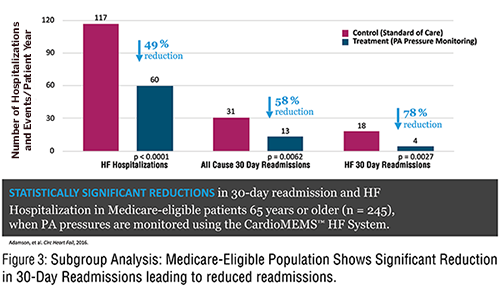
Figure 3 demonstrates the increased frequency of medication changes in the PA pressure guided therapy group.
Improving Intracardiac Pressures through Clinical Assessment
Data from CardioMEMs helps us as heart failure specialists appreciate that maintenance of appropriate volume status improves outcomes. Elevated PA pressures, from fluid retention, is associated with worsening dyspnea, which is the main reason patients seek emergency care. A goal for maintaining patient stability is to keep the pulmonary diastolic pressure below 22 mm HG.
In the absence of CardioMEMS data, what can our clinical assessment tell us about the numerous patients that we evaluate in the clinic? Data from the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) showed that two clinical findings were indicative of PCWP >22 mm Hg. One was the presence of orthopnea of two pillows or greater (sensitivity 86 percent and specificity 25 percent), and the other was estimated jugular venous pulse equal to or greater than 12 mm Hg (sensitivity 65 percent and specificity 75 percent).
Rales were not helpful, present less than 15 percent of the time, and 60 percent of patients with PCWP >22 did not have peripheral edema. 4, 5 Orthopnea or worsening orthopnea deserves more attention. Advise patients to call the office if they are increasing their “sleep angle.” Additionally, the absence of orthopnea at hospital follow-up is associated with a favorable hemodynamic state and has favorable prognostic implications. Elevated BNP (B type natriuretic peptide) levels are associated with increased cardiac pressures and may indicate volume overload. The BNP test has been used to diagnose heart failure and has prognostic implications. The question arose as to whether serial outpatient measurements of BNP would improve outcomes for patients.
The Guide-It study was performed by clinics with heart failure expertise in clinical assessment. This study failed to show benefit of serial BNP testing in reducing hospitalizations for heart failure.6
HFpEF and HFrEF
For treatment purposes, heart failure patients have been grouped into two phenotypes. Those with normal ejection fractions, and those with reduced left ventricular ejection fractions. Clinical trial data has only shown benefit of specific medications in those with reduced ejection fractions. A discussion of specific medications, dose titrations and how to implement combination therapy is beyond this article, but articles on guidelines and tips for management are included in the references. 7, 8
Patients with Heart failure With Preserved Ejection Fraction (HFpEF) have elevated cardiac pressures, particularly with activity, and may also have diminished cardiac output, either with rest or activity. Thus, patients with HFpEF have both diastolic and systolic dysfunction. Suspect HFpEF in patients with exertional dyspnea who have hypertension, obesity, type 2 diabetes mellitus, prior atrial fibrillation and abnormalities on echocardiogram such as evidence of pulmonary hypertension. A recent prediction model has been developed. (Figure 4)
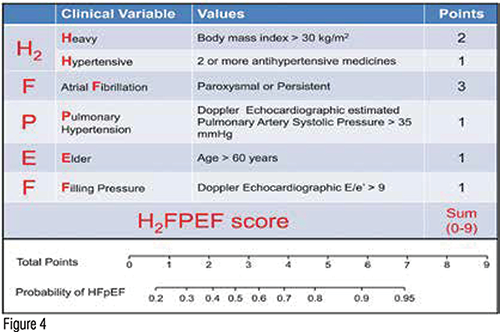
The majority of patients with Heart Failure with Reduced Ejection Fraction (HFpEF) have BMIs over 30, often over 35, and volume overload can be difficult to detect by neck vein exam. Additionally, resting BNP levels may be normal in these patients. A trial of diuretic therapy is indicated particularly in patients who have elevated sleep angles and worsening dyspnea.
Unlike HFrEF where there are specific medications to be given for optimal medical therapy, in HFpEF, numerous trials including those of B Blockers, ACE inhibitors, ARBs and other medications have all failed to show benefit. The TOPCAT study of spironolactone has been criticized, as subjects in one region of the world who were in a treatment arm did not have spironolactone metabolites in their urine, indicating they were not taking the drug. It has been argued that the results of the trial might have otherwise been positive.
In HFrEF, do not assume that a low ejection fraction means poor functional status. Marked fatigue in the patient with an LVEF of 20 percent and a favorable exam may well be unrecognized sleep apnea. One might also consider over diuresis. Checking for orthostatic pressures can be helpful.
Regardless of ejection fraction, a key goal is to achieve and maintain a euvolemic state through modification of diet and adjustment of diuretic therapies. This often includes the use of loop diuretics such as furosemide, bumetanide or torsemide. For patients showing inadequate response to higher doses of furosemide, consider the use of torsemide for its better absorption and longer half-life.
In diuretic resistance, consider the addition of a prn thiazide prior to the dose of the loop diuretic. Also recall that in the RALES trial, low-dose spironolactone was shown to benefit patients with HFrEF who were recently hospitalized.
Patients receiving loop diuretics and potassium supplements should be considered for low-dose spironolactone and discontinuation of potassium pills. Use of outpatient intravenous diuretics in the heart failure clinic may also be helpful in diverting the need for hospitalization.
Symptoms and physical findings of patients living with heart failure have poor correlation with left ventricular ejection fraction. Guideline-directed pharmacologic therapy is indicated if the patient phenotype is heart failure with reduced ejection fraction.
Patients with preserved ejection fractions and heart failure commonly have pro-inflammatory conditions such as obesity and diabetes mellitus. In both phenotypes, maintenance of a euvolemic state and control of blood pressure facilitates a more normal left ventricular end diastolic pressure. This results in improved symptoms and a reduction in heart failure hospitalizations, and likely improves long-term myocardial function.
Clinical assessment in the office as well as patient education on self-management can prevent destabilization in most patients. For patients with persistent NYHA class III symptoms and recent need for hospitalization, remote pulmonary artery pressure monitoring reduces hospitalization because of timely medication adjustment. Trials are ongoing to determine if remote management using multi-sensor data from implantable resynchronization pacemakers or ICDs can improve outcomes.

